

TYPES OF
RECTAL
SPACERS
Hydrogel Spacer vs PEG Hydrogel Spacer
All Hydrogel Spacers are Not Created Equal
There are three available hydrogel spacers used to protect the rectum during prostate cancer radiation. It is important to know the differences between them and how the material and control of each impacts the protection of healthy organs at risk of radiation exposure.1,2
Why is physician-controlled rectal spacer placement important?
Hydrogel spacers are placed by your doctor in between the prostate and the rectum, utilizing transrectal ultrasound (TRUS) medical imaging. The more control your doctor has over the placement of the spacer, the more personalized protection you may receive. Barrigel is the only hydrogel spacer that remains sculptable and highly visible throughout the procedure, allowing your physician to take their time and tailor the implant to your specific anatomy under constant image guidance. PEG hydrogel spacers polymerize in 8-15 seconds and have poor visibility on TRUS, offering limited control over final implant placement.3-5
Controllable Hydrogel Spacer vs. Uncontrollable PEG Hydrogel Spacer
Barrigel hydrogel spacer is made from Non-Animal Stabilized Hyaluronic Acid (NASHA®), a highly sculptable gel used in more than 50 million procedures in men, women, and children worldwide, most commonly known as the dermal filler, Restylane®.6 NASHA has been designed to create lifting power to separate tissue and is made from a material naturally found in the human body, hyaluronic acid. The sculptability and lifting power of the gel allow your doctor to have control to customize the placement for optimal protection of your rectum.
PEG hydrogel spacers are made from polyethylene glycol (PEG), which is often used as a tissue sealant and polymerizes (hardens) within 8-15 seconds of deployment.3-5 This time pressure constrains your doctor from the ability to take their time and sculpt the gel to be tailored to your anatomy, limiting the overall control they have to precisely place the implant, which can result in less-than-ideal rectal coverage.2
Controlled rectal spacing further protects you from the potential short-term and long-term effects of prostate cancer radiation.7
See the matrix below to review the features of controlled spacing.
How hydrogel rectal spacer material impacts patient safety
The control of implant placement is important for patient safety. Barrigel offers your doctor the ability to take their time placing the gel, while clearly seeing it on TRUS medical imaging, allowing for a more customized final implant placement.


How hydrogel rectal spacer material impacts symmetrical implant placement
Symmetrical rectal spacer placement is important in order to offer complete coverage of the rectum during prostate radiation. Barrigel hydrogel spacer is the only sculptable spacer available. Due to its customization properties, it has been shown to achieve symmetrical placement >95% of the time.1 As a result of the quick polymerization of PEG Hydrogels, symmetrical placement is less achievable. In the PEG Hydrogel FDA clinical trial, symmetrical placement was achieved 49% of the time.2
BARRIGEL
BARRIGEL
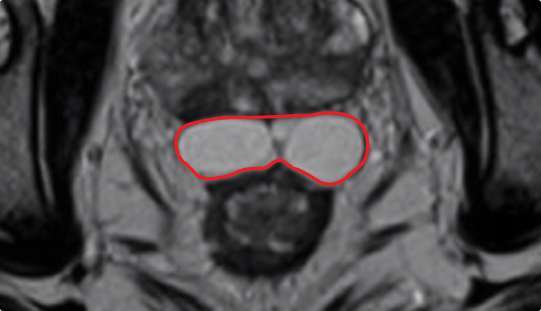
Implant Symmetry RESULTS1
>95%
PEG HYDROGEL
PEG HYDROGEL
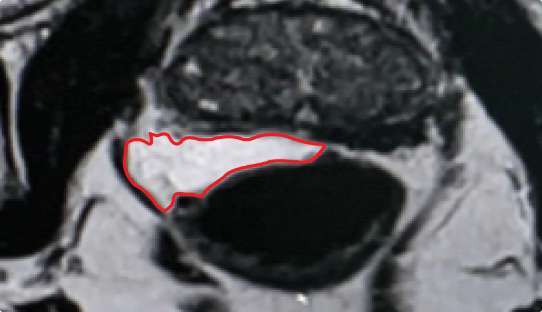
Implant Symmetry RESULTS2
49%


How Hydrogel Spacer Material Impacts Unwanted Radiation Exposure
The Barrigel (NASHA) rectal spacer was studied for the more aggressive hypofractionated radiation therapy for prostate cancer regimen and was clinically proven to significantly reduce radiation exposure by 85% in 98.5% of patients. 20% of patients received 100% reduction in unwanted radiation exposure to the rectum.7
The PEG Hydrogel spacer was studied for conventional radiation therapy for prostate cancer and was clinically proven to significantly reduce radiation exposure to the rectum by 73.3% in 97.3% of patients.8
How does Hydrogel Spacer Material Impact the Stability of Space Created?
The Barrigel (NASHA) rectal spacer is designed to maintain the space created for a longer period of time, ensuring full coverage throughout the entire length of your treatment. Stability is increasingly important if your radiation treatment needs to be delayed for any reason.
In the Barrigel FDA clinical trial, the space created by Barrigel was maintained at 3 months, with an average of ~2% loss of created space, while the implant itself begins to safely resorb into the body.7 In the PEG Hydrogel FDA clinical trial, the space created by the implant decreased by an average of ~30% at 3 months.8
How does Hydrogel Spacer Material Impact Patient Comfort?
The Barrigel (NASHA) rectal spacer remains flexible until it is safely resorbed by your body. The malleable nature of the gel allows the gel to move with the body naturally. There were zero reports of patient discomfort in the Barrigel pivotal trial, and there have been zero reports of patient discomfort in the more than 15,000 cases to date.7,10
Because PEG Hydrogel implants polymerize (harden), patients may feel rectal discomfort or pain from the spacer until it is safely resorbed by the body.9
What is an FDA-Reviewed, Randomized, Prospective Controlled Clinical Trial?
In an FDA-reviewed, randomized, controlled clinical trial, the experimental treatment is given to large groups of consenting patients in a carefully specified and closely monitored protocol. The goal of the study is for researchers to establish the treatment’s safety and effectiveness, monitor side effects, compare the treatment to the treatment in the control arm and collect information that will allow the treatment to be used safely. The study determines the efficacy and safety of the treatment being tested.
It is important to know that only two of three available rectal spacers have been studied in a rigorous FDA clinical trial. Ask your doctor whether your spacer has been proven safe and effective and the results of its trial.


References
1. King M, Svatos M, Chell EW. Assessment of NASHA Spacer Symmetry For Prostate Radiation Therapy.[ABSTRACT] Presented at American Brachytherapy Society Annual Conference, June 19, 2022.
2. Fischer-Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol. 2017;7(3):195-202.
3. What every urologist needs to know about SpaceOAR® hydrogel: How Augmenix is improving QOL for prostate cancer patients. Urology Times. May 2018.
4. Boston Scientific. Application Technique for SpaceOAR Vue™ Hydrogel: Dr. Asunció Hervas, Dr. José Dominguez. Accessed: January 30, 2023. https://www.bostonscientific.com/en-EU/medical-specialties/urology/hydrogel-spacing/learn-from-experts.html.
5. Boston Scientific. SpaceOAR™ System Indications, Safety, and Warnings. 2022. URO-819103-AB-JUN 2022
6. Restylane® celebrates 25 years of natural-looking results with its signature line of hyaluronic acid fillers. 2021. Available at: https://www.prnewswire.com/news-releases/restylane-celebrates-25-years-of-natural-looking-results-with-its-signature-line-of-hyaluronic-acid-fillers-301388779.html. Accessed Sept 30, 2021.
7. Mariados NF, Orio PF III, Schiffman Z et al. Hyaluronic acid spacer for hypofractionated prostate radiation therapy: A randomized clinical trial. JAMA Oncol. 2023: e1-e8.
8. Mariados N, Sylvester J, Shah Det al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971-977.
9. Aredkani MA, Ghaafari H. Optimization of prostate brachytherapy techniques with polyethylene glycol-based hydrogel spacers: A systematic review. Brachytherapy. 2020;19(13-23).
10. Data on file. Palette Life Sciences.