CONTROL MATTERS
CONTROL
MATTERS
Controlled Spacing with Barrigel Rectal Spacer What is controlled spacing in prostate cancer radiation therapy? The ability to create safe, anatomically-customized spacing for radiotherapy patients.1,2 Inherent Qualities: controllable sculptable flexible Controllable Barrigel rectal spacer gives you the ability to: Refine and adjust placement.1-4 in real-time.3,7 Sculptable Barrigel rectal spacer is sculptable, allowing customization throughout the procedure.1-3,5 Intentional Placement Barrigel rectal spacer allows for intentional placement, providing control over implant shape and placement.1-3,5 Controlled Spacing Results from the Barrigel Pivotal Trial.1 98.5% of patients achieved an average 85% dose reduction to the rectum (rV54) 20% of patients achieved 100% dose reduction to the rectum (rV54) Barrigel spacer was proven superior in the reduction of acute grade 2+ GI toxicity compared to control Zero (0) reports of implant migration For full study results please visit Barrigel.com IN RECTAL SPACING CONTROL MATTERS. References: Mariados NF, Orio PF III, King MT et al. JAMA Oncol (2023).*§ Svatos M, Chell E, Low DA et al. Med Phys (2024).*§ Gejerman G, Goldstein MM, Chao M et al. Pract Radiat Oncol (2023).§ Barrigel Injectable Gel Instructions for Use (2022). Williams J, Mc Millan K, Chao M et al. J Med Imaging Radiat Sci (2022).§ * Study sponsored by Palette Life Sciences, now part of Teleflex. § One or more of the authors are paid consultants of Teleflex.




SAFETY AND EFFICACY
MATTER
SAFETY AND EFFICACY
MATTER
Barrigel spacer is
proven superior
in the reduction of acute grade 2+ GI toxicity compared to control.1
Throughout the Barrigel Pivotal Trial, there were
zero
Barrigel-related
adverse events.1
SYMMETRY MATTERS
SYMMETRY
MATTERS
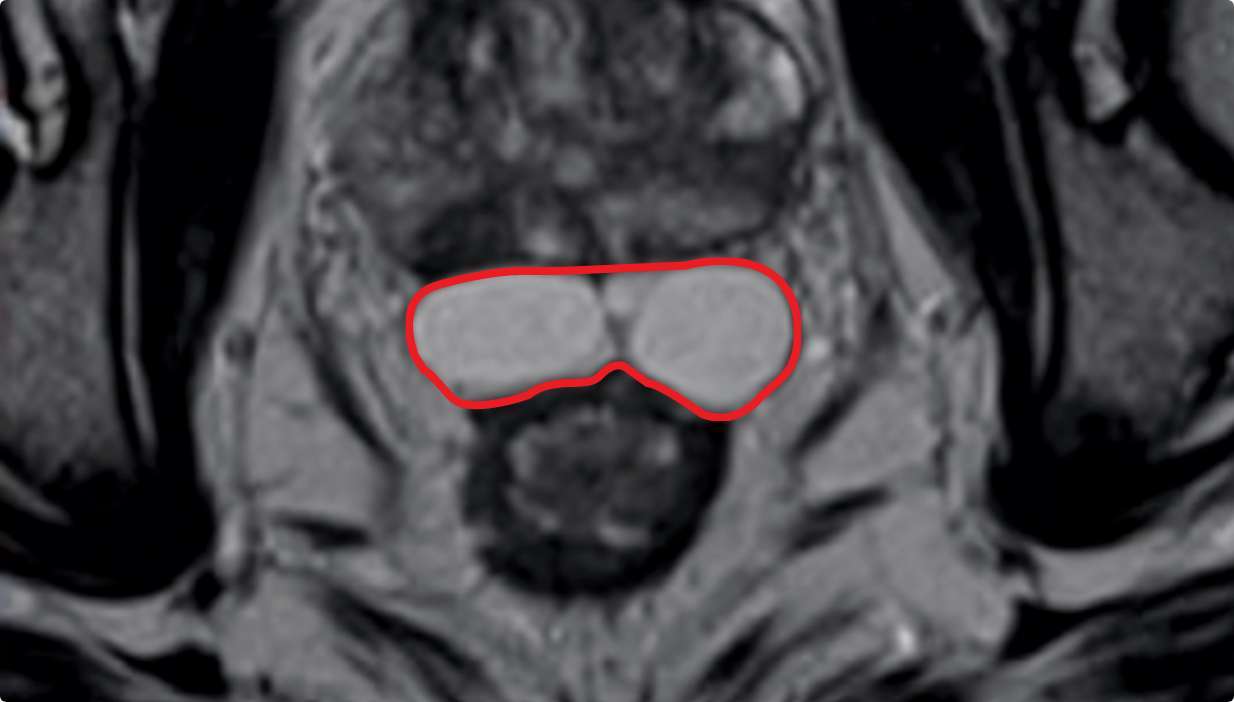
Results may vary.
STABILITY MATTERS
STABILITY
MATTERS

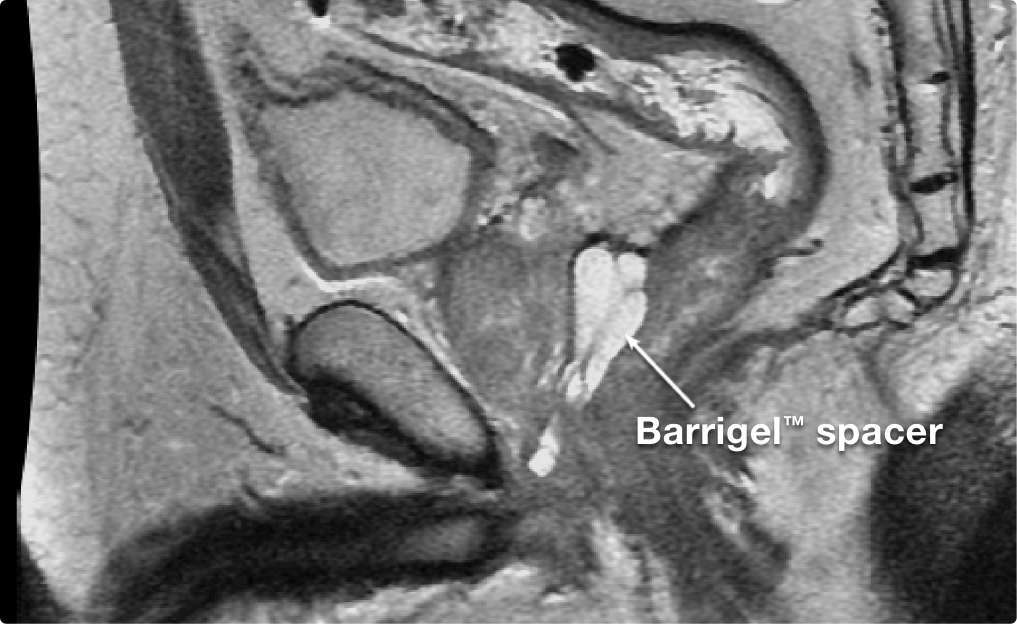
Results may vary.
References
1. Mariados NF, Orio PF III, King MT et al. JAMA Oncol (2023).†§
2. Svatos M, Chell E, King MT et al. Med Phys (2024).†§
3. Williams J, Mc Millan K, Chao M et al. J Med Imag Radiat Sci (2022).§
4. Gejerman G, Goldstein MM, Chao M et al. Pract Radiat Oncol (2023).§
5. Data on file. As of 4/01/2025.
† Study sponsored by Palette Life Sciences, now part of Teleflex.
§ One or more of the authors are paid consultants of Teleflex.