

CLINICAL
RESULTS
Proven Safe & Effective
At Reducing GI Toxicities
in patients undergoing radiation for prostate cancer.1
Clinically Proven:
Optimal Protection
for the rectum, reducing rectal side effects.1
OVERVIEW OF RECTAL SPACER TRIALS1,4,6
OVERVIEW OF RECTAL
SPACER TRIALS1,4,6
Results from different clinical studies are not directly comparable.




RCT = Randomized Controlled Trial
* Dosimetric analysis was based upon 81 Gy in 1.8 Gy fractions.
** 2.3% (5 patients) missing primary safety results due to insufficient follow-up.
† In a separate, directly analogous secondary analysis of data from the Barrigel pivotal trial, Barrigel symmetry results were compared to those of SpaceOAR, as reported in Fischer-Valuck BW et al. Pract Radiat Oncol (2017), using the same methodology used in that study. Information provided for educational purposes only. No head-to-head study has been performed. Results from different clinical studies are not directly comparable.


Barrigel Pivotal Trial Overview & Results:
TREATMENT PROTOCOL
TREATMENT
PROTOCOL
BENEFITS OF
HYPOFRACTIONATION
BENEFITS OF
HYPOFRACTIONATION
Hypofractionated radiation therapy (HFRT) – effective and more convenient
The utilization of HFRT has dramatically increased in recent years.7 While this modern form of radiation allows for higher doses given in fewer fractions, the rectum remains at risk for exposure. Given that HFRT has been associated with greater acute grade 2+ GI toxicity than conventionally fractionated radiation therapy (CFRT), rectal spacing may address a clinically important need for patients receiving HFRT.1
The Barrigel Pivotal Trial is the first and only FDA-reviewed randomized controlled study of rectal spacing that exclusively used hypofractionated radiation therapy.1,2


EFFICACY MATTERS
EFFICACY MATTERS
Barrigel spacer is proven effective at achieving a clinically significant reduction in radiation dose to the rectum, leading to fewer rectal side effects1
In the Barrigel pivotal trial, 98.5% of patients met the primary endpoint of achieving at least a 25% reduction in rectal V54 Gy* (p<0.001)1
*54 Gy is 90% of 60 Gy
reduction of acute grade 2+ GI toxicity compared to control1
- RADIATION PROCTITIS
- DIARRHEA
- HEMORRHOIDS
SAFETY MATTERS
SAFETY MATTERS
In the Barrigel pivotal trial, there were:1
ZERO Barrigel spacer-related adverse events
ZERO Peri-procedural events
ZERO Reports of rectal fullness
ZERO Patient complaints of device-related pain or discomfort


ADVANCED CONTROL
OVER PLACEMENT1,3,8,9
ADVANCED CONTROL OVER
PLACEMENT1,3,8,9
BARRIGEL SPACER
BARRIGEL SPACER
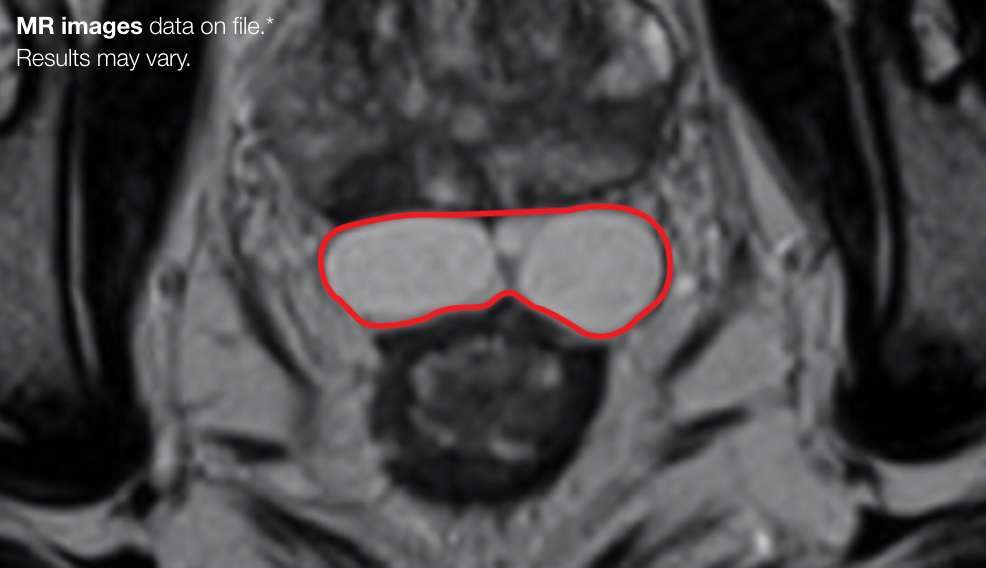
Implant Symmetry RESULTS3**
95.6%
Pivotal Trial Patients; % of implants centered on prostate midline
PEG HYDROGEL
PEG HYDROGEL
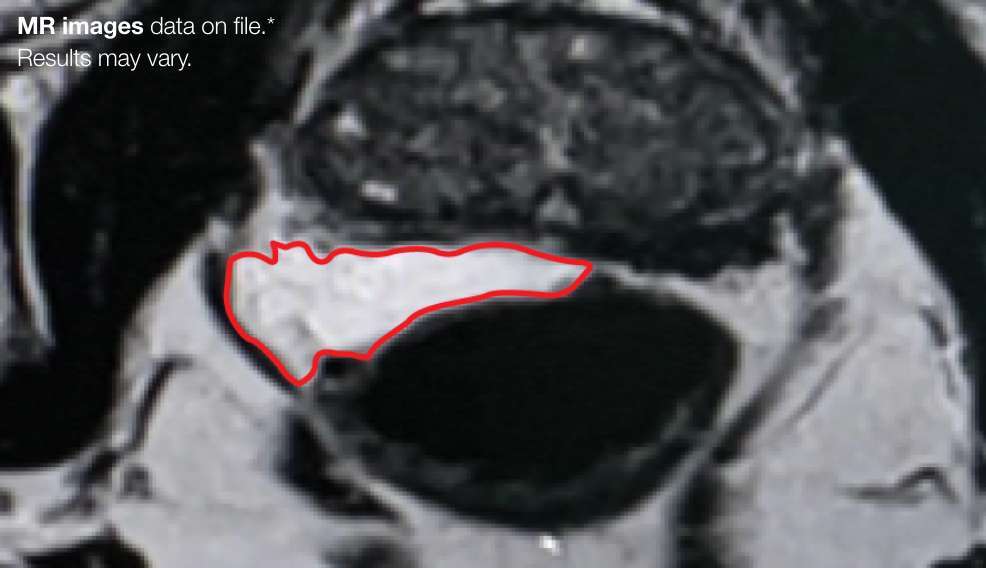
Implant Symmetry RESULTS5**
49%
Pivotal Trial Patients; % of implants centered on prostate midline
* Both rectal spacers were placed by the same physician.
** In a separate, directly analogous secondary analysis of data from the Barrigel pivotal trial, Barrigel symmetry results were compared to those of SpaceOAR, as reported in Fischer-Valuck BW et al. Pract Radiat Oncol (2017), using the same methodology used in that study. Information provided for educational purposes only. No head-to-head study has been performed. Results from different clinical studies are not directly comparable.
Does your rectal spacer provide you: Advanced control over implant placement. Does your rectal spacer provide you: The ability to sculpt with no time constraints. Does your rectal spacer provide you: Ninety-five point six percent symmetrical implant placement. In rectal spacing, control matters. References. Mariados N F, Orio P F the third, King M T et al. Implant test symetry results: ninety five point six (Barrigel) vs forty nine percent (PEG Hydrogel) JAMA Oncology, 2023. Svatos M, Chell E, King M T et al. Medical Physics, 2024. Gejerman G, Goldstein M M, Chao M et al. Practical Radiation Oncology, 2023. Williams J, McMillan K, Chao M et al. Journal of Medical Imaging and Radiation Sciences, 2022. Fischer-Valuck B W, Chundury A, Gay H, Bosch W, Michalski J. Practical Radiation Oncology, 2017. Study sponsored by Palette Life Sciences, now part of Teleflex. Barrigel is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer. Barrigel is composed of biodegradable material and maintains space for the entire course of prostate radiotherapy treatment. Barrigel should only be administered by qualified and properly trained physicians with experience in ultrasound guidance and injection techniques in the urogenital pelvic area. As with any medical treatment, there are some risks involved with the use of Barrigel. Potential complications include pain, injection-related events, urinary retention, bleeding, constipation, rectal urgency, and other risks as described in the instructions for use. More information on indications, contraindications, warnings, and instructions for use can be found at Barrigel dot com. Caution: Federal law restricts this device to sale by or on the order of a physician.
ACHIEVE CONSISTENT RESULTS
WITH BARRIGEL SPACER3,8,9
ACHIEVE CONSISTENT RESULTSWITH BARRIGEL
SPACER3,8,9
First Barrigel Spacer Cases - Consecutive Patients (Same Day)
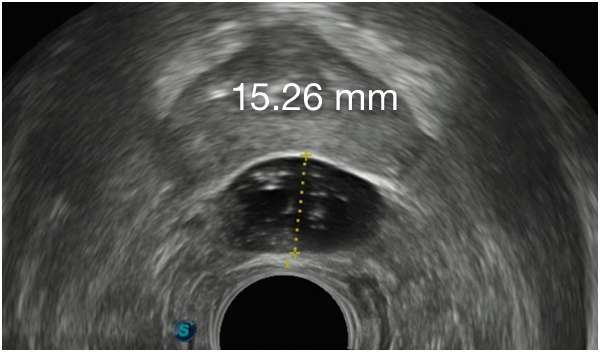
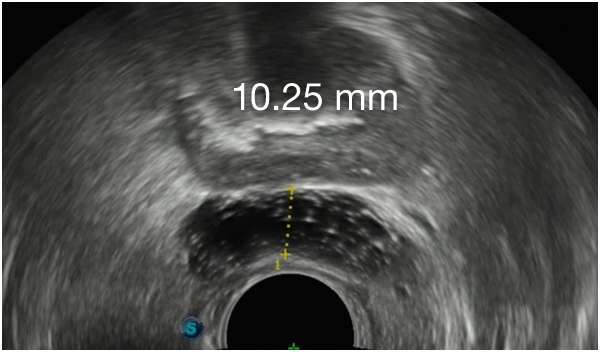
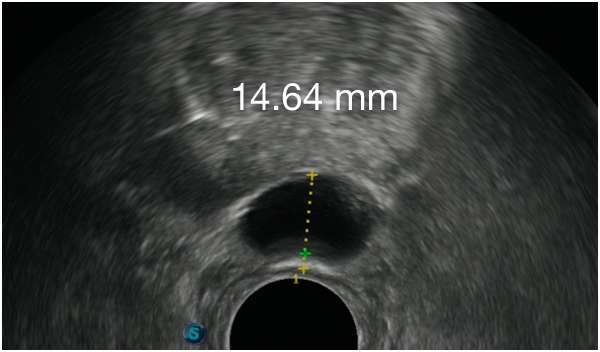
TRUS images courtesy of Daniel R. Welchons, MD
Urologist; New York, United States
Results may vary.
DR. WELCHONS’ INJECTION TECHNIQUE


IN RECTAL SPACING
STABILITY MATTERS
IN RECTAL SPACING STABILITY
MATTERS
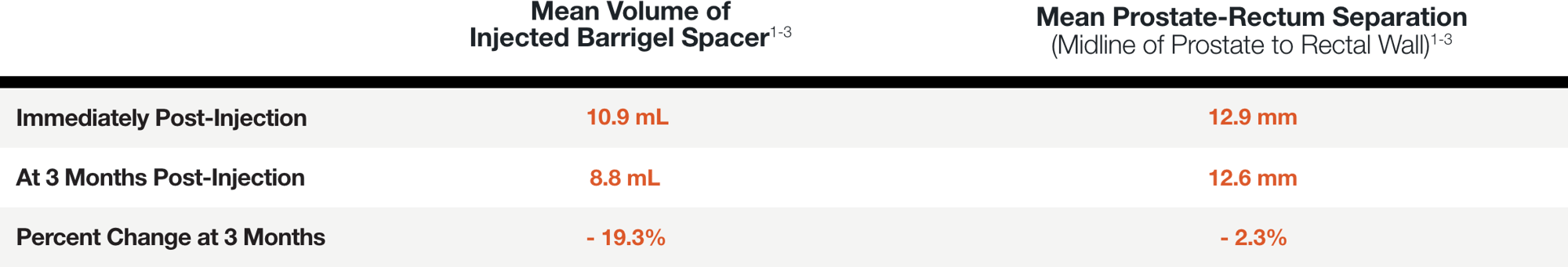
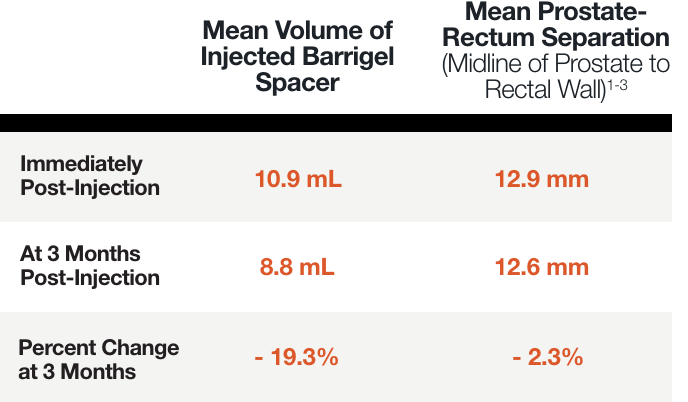
PATIENT EXAMPLE SHOWING
STABLE SEPARATION DURING RESORPTION



Results may vary.
MEAN PROSTATE-RECTUM SEPARATION (DIMENSIONAL STABILITY)
References
1. Mariados NF, Orio PF III, Schiffman Z, et al, JAMA Oncol (2023).‡§
2. Data on file. As of 4/01/2025.
3. Svatos M, Chell E, Low DA et al. Med Phys (2024).‡§
4. Mariados NF et al, Int J Radiat Oncol Biol Phys (2015).
5. Fischer-Valuck BW et al, Pract Radiat Oncol (2017).§
6. Song D et al, Int J Radiat Oncol Biol Phys (2024).
7. Dearnaley D, Syndikus I, Mossop H et al. Lancet Oncol (2016).
8. Gejerman G, Goldstein MM, Chao M et al. Pract Radiat Oncol (2023).§
9. Williams J, Mc Millan K, Chao M et al. J Med Imag Radiat Sci (2022).§
10. King MT, Svatos M, Orio PF III et al. Pract Radiat Oncol (2023).‡§
‡ Study sponsored by Palette Life Sciences, now part of Teleflex.
§ One or more of the authors are paid consultants of Teleflex.